Relevance
Newest
Oldest
X Clear Filters
Insights
-
Featured
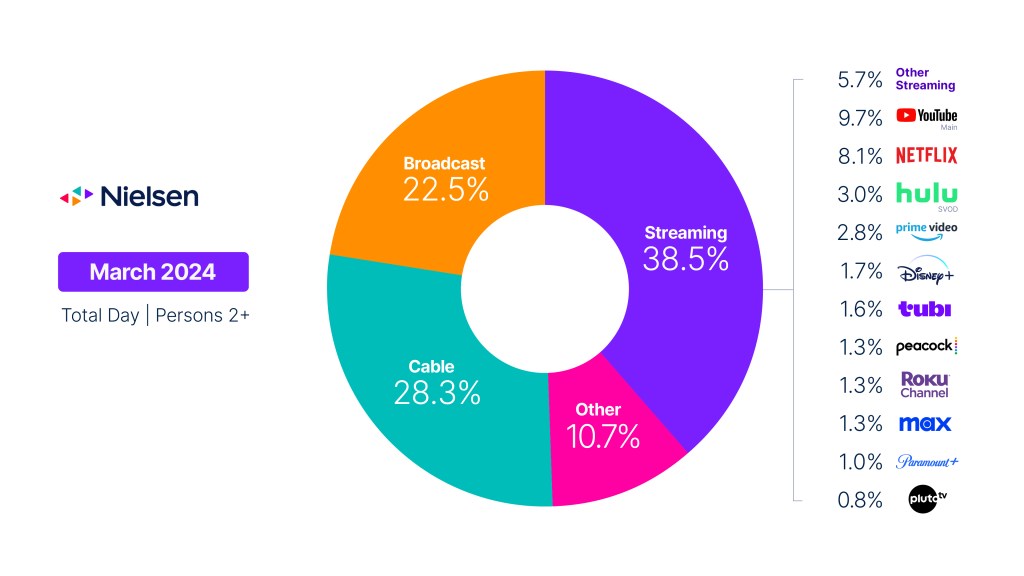
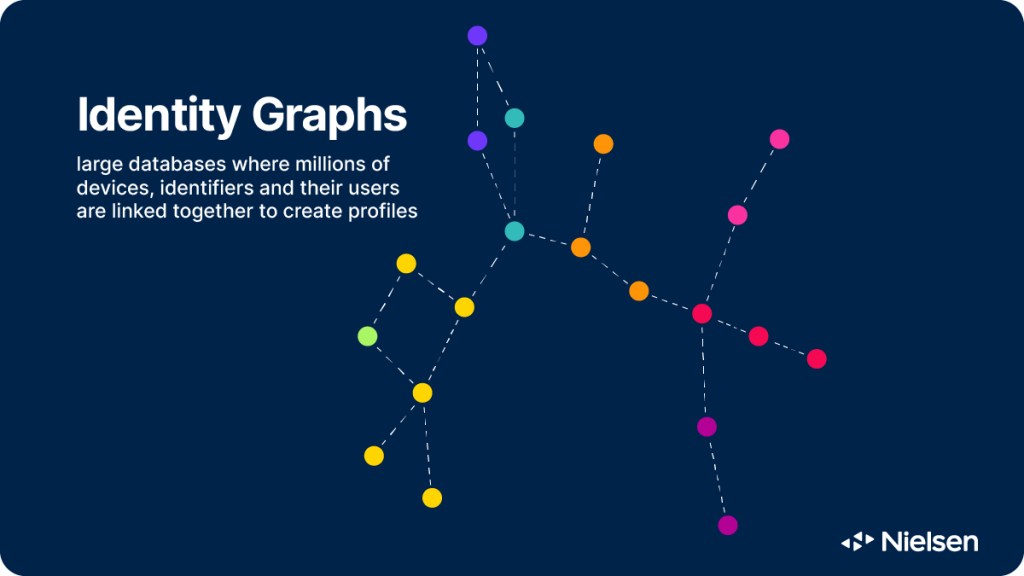
Need to Know: What’s an identity graph and why do marketers need them?
For consistent, comprehensive and comparable audience measurement across platforms, marketers need a robust ID system…
Marketing performance7m read